|
|



November 10, 2020
This report from the Missouri Hospital Association is designed to help you stay abreast of recent developments related to COVID-19.
Share our online form with individuals interested in receiving this update.
Dashboard Spotlight
Education
Hospital Operations
Legal & Regulatory Resources
Noteworthy
Testing, Reporting & Treatment
Vaccine Updates
|
|
Dashboard Spotlight
|
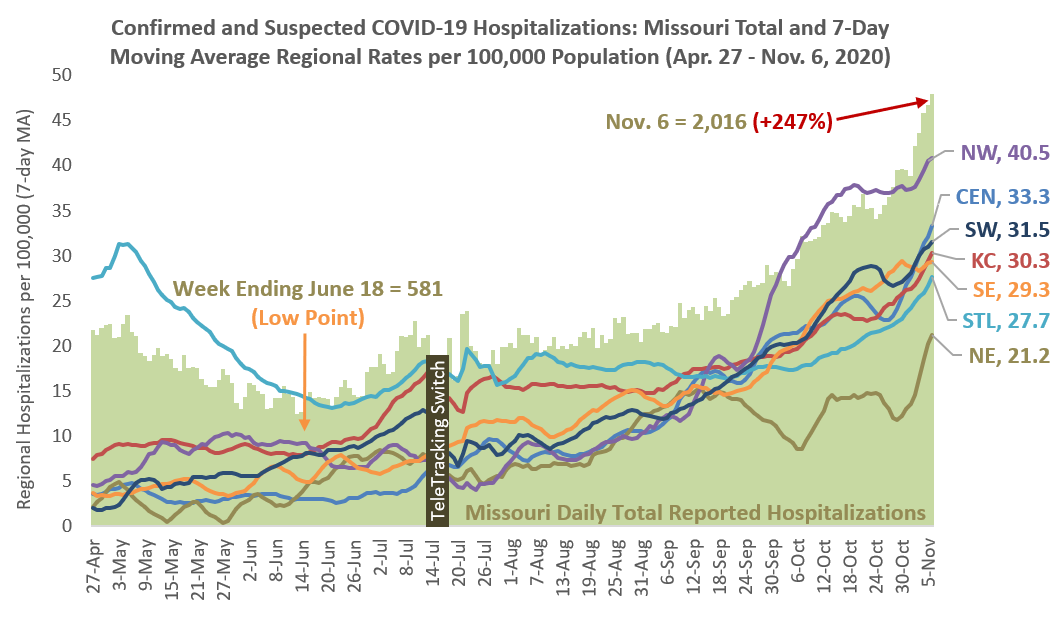
Statewide COVID-19 Hospitalizations Continue To Exceed Previous Records
Staff Contact: Jackie Gatz or Mat Reidhead
Statewide confirmed and suspected COVID-19 hospitalizations have continued to exceed previous records based on data reported to the U.S. Department of Health and Human Services TeleTracking system. With more than 2,000 COVID-19 patients reported for the first time in Missouri on Nov. 6, suspected and confirmed hospitalizations have increased 247% since the state’s lowest reported totals during the middle of June.
As community cases rise, COVID-19 has had a significant toll on the health care workforce, creating shortages due to quarantined or afflicted staff. Concurrently, hospitals are experiencing additional strain in inpatient volumes related to seasonal increases for respiratory and other illnesses while still serving pent-up demand from the spring pandemic-related shutdowns. Hospitals currently are strained but are responding with changes to expand capacity to care for all patients, including those with COVID-19, amidst reduced workforce. |
 |
|
HIDI Releases Hospital-Specific COVID-19 Dashboard
Staff Contact: Jackie Gatz or Mat Reidhead
The Hospital Industry Data Institute is pleased to announce the availability of new hospital-specific COVID-19-focused dashboards through HIDI Analytic Advantage®. The dashboards are designed to provide the following C-suite-level situational awareness on hospital-level trends.

- the submission of key COVID-19 data elements to the U.S. Department of Health and Human Services’ TeleTracking data portal
- a distillation of weekly compliance for your hospital, as reported by HHS
- operational impacts and recent hospital activity related to influenza- and COVID-19-like illnesses
Dashboards are available for download to authorized users of HIDI Analytic Advantage®.
- Location: Strategic Planning > Additional Resources
- File Name: MOxxxx_COVID-19_Dashboard_2020.MM.DD
- Documentation: MO0000_HIDI Hospital-Specific COVID Dashboard Documentation v1.1 2020.11.10.pdf
We trust this information will be helpful to you and your organization. If you need a user ID and password or have questions about accessing these dashboards, please contact hidi@mhanet.com.
Back To Top
|
|
Education
|
Aya Healthcare Hosts Webinar On Hiring
Staff Contact: Jill Williams
Aya Healthcare is hosting a complimentary webinar, “Healthcare Hiring Pulse Check: Keeping Up With Market Dynamics,” at 1 p.m. CST Wednesday, Nov. 11. Impacts of the COVID-19 pandemic on the health care workforce are widespread, leaving hiring leaders in a frenzy to add talent faster than ever while balancing budget, quality and a great degree of uncertainty. During the webinar, participants will discuss market factors contributing to hiring challenges, best practices for health care systems to conquer the challenges of today and preparing for the months ahead.
Back To Top
CMS Hosts Upcoming Stakeholder Engagement Calls
Staff Contact: Leslie Porth
The Centers for Medicare & Medicaid Services hosts its COVID-19 Office Hours Call twice a month on Tuesdays to provide hospitals, health systems and providers with an opportunity to ask questions regarding CMS’ temporary actions. The next call takes place at 4 p.m. CST Tuesday, Nov. 17. Participants can join via audio webcast, and use dial-in number 833-614-0820 with passcode 2491556. Call recordings and transcripts are posted on the CMS podcast page.
Back To Top
NAM Publishes Resources For Clinician Well-Being
Staff Contact: Jill Williams
Video recordings and speaker presentations now are available from the recent National Academy of Medicine’s Action Collaborative on Clinician Well-Being and Resilience virtual meeting. This event elevated the urgency of national action and mapped efforts needed to coordinate the long-term health and well-being of clinicians during and after the COVID-19 pandemic.
Back To Top
|
|
Hospital Operations
|
CDC Issues Guidance For COVID-19 Screening At Hospital Entrances
Staff Contact: Jane Drummond or Sarah Willson
The Centers for Disease Control and Prevention issued revised guidance for screening patients, visitors and workers arriving at a health care facility. The guidance provides multiple options for identifying COVID-19 symptoms, including electronic temperature surveillance, individual screenings, or self-assessment and reporting of symptoms. The guidance also provides information on factors that may impact accurate thermometer readings, resources for ensuring proper ventilation systems and access to FAQs about the use of personal protective equipment.
Hospital surveyors in other states previously indicated workers may not attest to their own temperature when arriving at work, based on CDC guidance. The revised guidance may help to clarify this issue.
Back To Top
CDC Releases Cleaning Resource For Health Care Environment
Staff Contact: Keri Barclay or Jessica Stultz
The Centers for Disease Control and Prevention released a new resource highlighting core components of environmental cleaning and disinfection in hospitals. The document aims to help health care organizations reduce the risk of infection from surfaces, and provides descriptions and examples of each of the six core components to ensure a clean patient care environment.
Back To Top
|
|
Legal & Regulatory Resources
|
CMS Issues New CLIA Survey Guidance
Staff Contact: Sarah Willson
The Centers for Medicare & Medicaid Services released new guidance to state survey directors regarding an optional process for Clinical Laboratory Improvement Amendments surveys. QSO-21-04-CLIA explains the flexibility to perform remote CLIA recertification surveys during the COVID-19 public health emergency.
Back To Top
|
|
Noteworthy
|
Study Finds Greater Severity, Disability In COVID-19 Strokes
Staff Contact: Keri Barclay
A recent University College London and UCL Hospital study found that COVID-19-associated ischemic strokes — caused by an obstruction of blood vessels in the brain — are more severe and more likely to result in disability or death than non-COVID-19 strokes are. Strokes were more likely to be severe in infected patients, and stroke patients only were half as likely to leave the hospital without disability as those without COVID-19. In addition, COVID-19 stroke patients also had significantly higher rates of in-hospital mortality.
Back To Top
|
|
Testing, Reporting & Treatment
|
FDA Authorizes First COVID-19 Neutralizing Antibody Test
Staff Contact: Jackie Gatz or Keri Barclay
The U.S. Food and Drug Administration on Friday issued an emergency use authorization for the first serology test to identify individuals with neutralizing antibodies from recent or prior infection with the virus that causes COVID-19. Neutralizing antibodies have reduced the infection of cells in a laboratory setting, but researchers still are studying their effect in humans. The FDA previously issued EUAs for serology tests that detect binding antibodies, which do not necessarily reduce cell infection. This test may tell us about potential immunity; however, patients should not interpret the results as having any level of immunity from the virus, according to Tim Stenzel, M.D. from the FDA.
Back To Top
FDA Grants EUA For Antibody Drug Bamlanivimab
Staff Contact: Jackie Gatz or Leslie Porth
The U.S. Food and Drug Administration on Monday granted an emergency use authorization to Eli Lilly for the antibody drug Bamlanivimab for people 12 and older with mild or moderate COVID-19 not requiring hospitalization. It is a one-time treatment given through an IV. Bamlanivimab should be given as soon as possible after positive results of direct SARS-CoV-2 viral testing and within 10 days of symptom onset. The government has signed an agreement to purchase 300,000 vials of the drug. A fact sheet for health care providers is available, and additional information will be shared as it becomes available.
Back To Top
DHSS Releases Health Update On Measures To Limit False Positive COVID-19 Antigen Tests
Staff Contact: Jackie Gatz or Keri Barclay
Today, the Missouri Department of Health and Senior Services issued a health update with recommended measures to limit false positive results with COVID-19 antigen tests. The update stressed the importance of following the manufacturer’s instructions when using antigen tests for COVID-19. The U.S. Food and Drug Administration issued a letter of concern regarding the potential for false positive results with antigen tests for the rapid detection of SARS-CoV-2, the virus that causes COIVD-19. Failure to strictly adhere to the manufacturer’s instructions and the following guidance provided by the FDA may lead to an increase in false positive test results, which can have a substantial negative impact on the community.
Back To Top
|
|
Vaccine Updates
|
Pfizer Announces Vaccine Candidate Against COVID-19
Staff Contact: Jackie Gatz
Yesterday, Pfizer and Biontech announced a successful vaccine candidate against COVID-19 from their Phase 3 Study. “Today is a great day for science and humanity. The first set of results from our Phase 3 COVID-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent COVID-19,” said Dr. Albert Bourla, Pfizer Chairman and CEO. The vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis. A submission for emergency use authorization to the U.S. Food and Drug Administration is planned for soon after the required safety milestone is achieved, which currently is expected to occur in the third week of November.
Back To Top
|
 |